
Measure the mechanical properties of pharmaceutical packaging
Learn how a Texture Analyser can assess the mechanical properties of pharmaceutical packaging through a range of different testing approaches.

Why measure the mechanical properties of pharmaceutical packaging?
The measurement of mechanical properties in pharmaceutical packaging is a vital practice within the pharmaceutical industry, encompassing numerous applications, sample types, setups, and geometries. This versatile approach finds relevance across various pharmaceutical testing scenarios, such as oral and transdermal delivery systems, as well as packaging.
The objective is to ensure the protection of pharmaceutical products during transit, use, and storage over a reasonable timeframe. A Texture Analyser serves as a valuable tool for assessing various aspects of pharmaceutical packaging, simulating shipping conditions through force cycle tests that replicate the product's repeated loading during transportation. Storage testing involves timed compression to mimic the effects of stacking products on a shelf. Additionally, assessing the strength of bottle caps and packaging seals are crucial.
When navigating the complex landscape of pharmaceutical product development and quality control, which involves diverse regulatory requirements, innovative ingredients, evolving packaging formats, changing consumer demands, and financial constraints, the role of mechanical property measurement becomes even more critical. In today's demanding environment, achieving long-term commercial success and perfecting pharmaceutical products necessitates a meticulous understanding of packaging behaviour and performance under real-world conditions.






How a Texture Analyser can be applied to pharmaceutical packaging mechanical property measurement?
Pharmaceutical packaging plays a crucial role in ensuring drug safety, efficacy, and longevity. Given its importance, understanding and optimising the mechanical properties of such packaging becomes paramount. Implementing a Texture Analyser to measure these properties in the context of pharmaceutical packaging offers multiple advantages:
- Integrity and safety: Testing for tensile strength, tear resistance, and sealing quality ensures that pharmaceutical packaging is robust and tamper-evident, safeguarding the enclosed product.
- Barrier properties assessment: The efficacy of some drugs is contingent upon being shielded from moisture, oxygen, or light. By analysing the mechanical resistance of barrier coatings or layers, manufacturers can ensure optimal protection.
- Child-resistant and senior-friendly designs: Packaging must often strike a balance between being difficult for children to open while remaining accessible to seniors. Evaluating opening forces and mechanisms helps achieve this balance.
- Predictive modelling and shelf life: By understanding how packaging materials degrade or change over time and under different conditions, manufacturers can predict shelf life and make informed storage recommendations.
- Regulatory compliance: Regulatory bodies often have stringent standards for pharmaceutical packaging to ensure drug safety. Regular testing confirms adherence to these standards.
- Innovation in design: As the pharmaceutical industry evolves, so do packaging needs. Testing novel materials or designs, like eco-friendly packaging or smart packaging solutions, ensures they meet required mechanical specifications.
- Economic and sustainability evaluations: By determining the precise mechanical requirements for specific drugs or environments, unnecessary over-engineering or material usage can be avoided, leading to cost savings and sustainability benefits.
- Transport and distribution suitability: Evaluating properties like compression strength or impact resistance ensures that packaging can withstand the rigors of transportation without compromising product integrity.
- User experience: The ease of opening, resealing, or dispensing from pharmaceutical packaging influences patient compliance. By gauging these aspects, manufacturers can enhance the end-user experience.
- Quality control and assurance: Regular mechanical testing across batches ensures consistent quality, preventing costly recalls or reputational damage.
- Compatibility testing: Certain drugs may interact with packaging materials over time. While this primarily concerns chemical interactions, mechanical changes in the packaging can also indicate potential issues.
- Feedback for material suppliers: Consistent testing can provide feedback to packaging material suppliers, ensuring alignment with the required specifications for pharmaceutical applications.
Employing a Texture Analyser in the development and manufacture of pharmaceutical packaging offers a robust, empirical approach to ensuring safety, compliance, user-friendliness, and efficiency. This data-driven method aids in producing packaging that not only preserves drug efficacy but also aligns with logistical, economic, and patient-centric considerations.
Typical measurements
For pharmaceutical packaging, it's crucial to ensure both the protection of the enclosed product and user-friendliness for the consumers.
Here are the mechanical properties that can be measured for pharmaceutical packaging using a Texture Analyser:
Tensile strength
This determines the maximum force the packaging material can withstand while being stretched before it breaks.
Flexural strength
Measures the material's resistance to deformation under bending, critical for packaging that might be folded or bent during use.
Puncture/penetration resistance/burst force
Assessing the puncture force or pressure a blister pack, blood bag or sachet can withstand before rupturing.
Compressibility
Measures the packaging material's response to compressive forces and its recovery post-compression.
Child-resistant mechanisms
Assessing the force or motion required to open child-resistant caps or blister packs.
Adhesion testing
For labels or adhesives used in the packaging, determining their adherence strength to the packaging surface.
Friction and slip resistance
Evaluates how the surface of the packaging interacts with other surfaces, impacting stackability and handling.
Peel strength
Measures the force needed to open peelable lids, sachets, or strips, ensuring they're secure yet user-friendly.
Tear resistance
Evaluates the force required to initiate or continue a tear in the packaging, especially important for easy-open designs.
Compression strength/Crush resistance
Determining the compressive strength of a bottle, carton, or other rigid packaging forms.
Snap fit analysis
For caps or closures that snap into place, evaluating the force required for attachment and detachment.
Syringeability
Measuring the force required to depress the plunger of prefilled syringes or auto-injectors.
Seal strength
It's essential to quantify the integrity of seals in blister packs, sachets, or other sealed packaging to ensure product protection.
Spring-back
Relevant for flip caps or press-top lids, this tests the ability of the packaging to revert to its original position after being opened.
Evaluating these mechanical properties ensures pharmaceutical packaging is not only protective and compliant with regulations but also user-centric, ensuring ease of access and use for patients.
Typical product test and graph
Case studies
Whether its providing the solution for Lubrizol Advanced Materials to characterise their homogeneous film compositions for medical and pharmaceutical applications, giving Queen’s University Belfast the ability to explore the stability and integrity of MN patches stored in different primary packaging under accelerated storage conditions or offering a method to ensure blister pack extraction force is optimised, a Texture Analyser is adaptable and flexible in its application to measure the bespoke mechanical properties of your product and then enable its quality to be controlled in your manufacturing to guarantee consistency and customer satisfaction.
With deep expertise in the physical property measurement of materials, we’re well equipped to support innovation in this sector – just ask our customers.
Probes and attachments for measuring the mechanical properties of pharmaceutical packaging
A wide range of probes and attachments can be integrated with our instruments, allowing testing to be precisely adapted to the material or product under evaluation. Applications include compression tests to compare blister packaging removal force, puncture tests to assess film burst strength, or tensile tests to measure and control the seal strength of packaging.
Over the years, we have collaborated with leading scientists and organisations across diverse industries to design and refine attachments that meet highly specific testing requirements. When a suitable solution does not already exist, we develop one – expanding our portfolio of Community Registered Designs and reinforcing our commitment to innovation in solving complex testing challenges.
The examples provided illustrate a selection of specialised attachments and commonly performed measurements in this application area. This list is not exhaustive; a wide range of additional options are available for the testing of food packaging. All instruments in the Texture Analyser range can be used to perform the tests described.
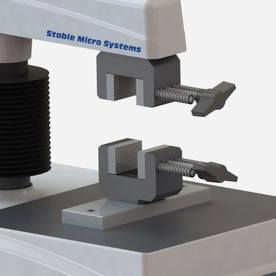
Tensile Grips

Self-tightening Roller Grips

Universal Peel Rig

Blister Pack Support Rig

Friction Rig

Horizontal Friction System

Film Support Rig

Sachet/Tube Extrusion Rig
Test methods
Exponent Connect software includes a comprehensive range of test methods for for pharmaceutical packaging , all instantly accessible at the click of a button. We streamline your texture testing process, ensuring faster access to methods and ready-to-use analysis files for your product properties.
Using the Texture Analyser for new pharmaceutical packaging material and product ideas
Pharmaceutical packaging plays a pivotal role in ensuring the stability, integrity, and safety of the drugs enclosed. Research in this sector is driven by a need for improved patient adherence, extended shelf life, tamper evidence, child resistance, and environmental sustainability. Here are some emerging trends and innovations in pharmaceutical packaging:
Child-resistant and senior-friendly packaging
Developing packaging solutions that are easy for adults to open but difficult for children, to prevent accidental ingestion.
Eco-friendly packaging
Emphasis on recyclable materials, biodegradable plastics, and reduced packaging material to minimize environmental impact.
Blister packs with enhanced barrier properties
Protection against moisture, light, and oxygen, extending the shelf life of the drug.
Desiccant integration
Including moisture-absorbing components within packaging to ensure drug stability.
Smart polymers
Responsive polymers that can change properties in response to environmental factors such as pH, temperature, or light, ensuring the stability of sensitive drugs.
Tamper-evident packaging
Packaging that clearly indicates if it has been opened or tampered with.
Unit dose packaging
Single-use packaging to ensure accurate dosing and reduce medication errors.
Auto-injectors and prefilled syringes
Packaging for biologics and vaccines that allow for easy self-administration.
3D printing
Customised packaging solutions, especially for clinical trial samples or niche drugs.
Antimicrobial packaging
Materials embedded with antimicrobial agents to prevent contamination.
Recent research
The following studies demonstrate how the Texture Analyser supports innovation in pharmaceutical packaging product development.
- Preparation and Characterization of Ecofriendly food Packaging Material by Smooth Hound Skin Gelatin Films/TiO2
- Dispensing Ease of Toothpaste from Squeezable Tubes
- From the laboratory to the end-user: a primary packaging study for microneedle patches containing amoxicillin sodium
- Effect of Rheology and Cooling on Paste Extrusion Using Texture Analysis
- Smooth Hound Skin Gelatin-TiO2 Films: Physicochemical and Structural Characterization
- A simplified stability assessment for selection of a suitable package for microporous osmotic tablets
- Morphological, barrier and thermo-mechanical properties of high-pressure treated polylactide graphene oxide reinforced composite films
Within powder and pharmaceutical packaging product R&D, the Texture Analyser is an indispensable tool, driving the optimisation of packaging design, strengthening safety measures, improving user convenience, and ensuring the efficacy of pharmaceutical and powder packaging solutions.
By deploying a Texture Analyser, pharmaceutical companies can confidently meet regulatory requirements, safeguard product integrity, and deliver patient-centric packaging. This advanced analytical instrument delivers precise, quantifiable data that supports the optimisation of packaging materials, design, and sealing methods – guaranteeing safety, reliability, and an enhanced user experience.




